Abstract
Background: Hypomethylating agents (HMA) are the only approved agents to treat CMML. Phase III trials for HMA included few patients (pts) with myelodysplastic CMML (MD-CMML) but excluded pts with myeloproliferative CMML (MP-CMML). In pts eligible for Dacota trial (Itzykson et al Blood (2020) 136 (Supplement 1): 53-54), the reported outcomes have been variable in those treated with HMA. Therefore, we sought to define the outcomes of pts with CMML treated with HMA stratified by disease subtype.
Methods: We analyzed a cohort of 286 pts with CMML treated with HMA at our institution from 3/2004 to 11/2019. Pts treated with HMA were classified into MD-CMML and MP-CMML groups (gp). Response rates and outcomes were analyzed by disease subtype and risk categories.
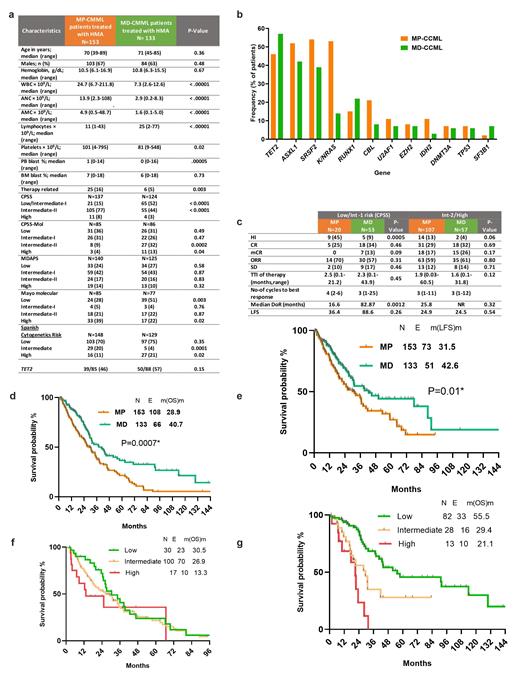
Results: Among the 286 pts, 153 had MP-CMML (53%) and 133 had MD-CMML (47%). Baseline characteristics are listed in Figure 1a. The median age of the entire cohort was 71 yrs (range, 39-89) with 71% aged ≥65 yrs. Compared to pts with MD-CMML, pts with MP-CMML treated with front line HMA were more likely to have higher percentage of circulating blasts (P<0.005), higher platelet count (P=0.02) and therapy related CMML (P=0.03). Pts with MP-CMML were more likely to be higher CPSS risk (Int-2+High) than those with MD-CMML (P <.0001). Entire cohort was enriched with TET2 (51%), ASXL1 (47%), SRSF2 (47%), KRAS/NRAS (37%) mutations with MP-CMML pts more likely to harbor KRAS/NRAS mut (P <.0001), CBL mut(P=0.04), and IDH2 mut (P=0.04) (Figure 1b).
In the entire cohort, the ORR was 61% with 47% CR and when stratified by CPSS risk, the ORR was 70% and 60% in lower and higher risk categories, respectively. In the lower risk gp, pts with MP-CMML had an ORR of 70% with 25% CR and were more likely to achieve hematological improvement (HI) compared to MD-CMML (P=0.0005) (Figure 1c). In the higher risk gp, ORR was similar between MP and MD-CMML (59% vs 61%, P=0.8). The median number of cycles required to achieve the best response was 3 (range, 1-26) regardless of risk gp or disease subtype with no difference in the time to initiation of therapy. In the entire cohort, 37 (13%) pts underwent allogeneic stem-cell transplant.
At a median follow up of 54.5 m, the median duration of response (DoR) was 33.3 m (range,0.1-109.3 m). Compared to pts with MP-CMML, pts with MD-CMML had longer durable responses (16.6 vs 82.9 m, P=0.001) with a longer relapse-free survival (RFS: 10.6 vs 20.2m, p=0.02). The median overall survival (OS) and leukemia free survival (LFS) in the entire cohort were 31.7 m (range, 1.1-163 m) and 35.9 m (range, 0.7-148 m), respectively. Compared to pts with MP-CMML, those with MD-CMML have longer OS (40.7 vs 28.9 m, P=0.0007) (Figure 1d) and LFS (42.6 vs 31.5 m, P=0.01) (Figure 1e).
When stratified by low, intermediate and high CPSS risk, the median OS in MP-CMML were 30.5 m, 26.9 m and 13.3 m (Figure 1f), and in MD-CMML were 55.5 m, 29.4 m, 21.1 m, respectively (Figure 1g). In those pts evaluable for CPSS-Mol (n=171), the median follow up was 29.7 m (range,1.2-107.5 m). When stratified by CPSS-mol risk, pts with low/Int-1 risk MP-CMML (36.7 vs 22.0 months, P=0.05) and MD-CMML had significantly longer OS (NR vs 31.2 m, P=0.04) compared to those with Int-2/high risk. Neither RAS/MAPK pathway mutations (34.6 vs 31.7 m, P=0.44) nor TET2/ASXL1 co-mutations (34.6 vs 31.7 m, P=0.88) had an impact on survival in pts with either MP or MD CMML treated with HMA.
We then evaluated the outcomes of pts with MP-CMML who met inclusion criteria for the Dacota trial to determine the response and survival outcomes in this subgroup. The ORR (58% vs 63%, P=0.41) and CR rates (26% vs 30%, P=0.48) were similar between pts who did or did not meet the eligibility criteria for the Dacota trial. However, in pts who met the eligibility criteria for the Dacota trial, the median DoR was significantly shorter (16.6 vs 82.9 m, P<0.0001). The high risk Dacota trial population also had significantly decreased median OS (25.2 vs 38.1m, P=0.0004) and LFS (24.9 vs 39.3m, P=0.003) than those who did not meet the eligibility criteria.
Conclusion: HMA therapy in CMML is associated with similar responses between disease subtypes and risk categories. Despite shorter survival among MP-CMML, even in higher risk pts meeting criteria for Dacota trial, HMA therapy is associated with median OS of 2 years. We did not observe influence of RAS/MAPK pathway mutations and TET2/ASXL1 co-mutations in survival outcomes of pts with either MP or MD CMML treated with HMA.
Sasaki: Pfizer: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding. Kadia: Dalichi Sankyo: Consultancy; Genentech: Consultancy, Other: Grant/research support; Jazz: Consultancy; Liberum: Consultancy; AstraZeneca: Other; Pfizer: Consultancy, Other; Cure: Speakers Bureau; AbbVie: Consultancy, Other: Grant/research support; Aglos: Consultancy; Amgen: Other: Grant/research support; Pulmotech: Other; Astellas: Other; Cellonkos: Other; Novartis: Consultancy; Sanofi-Aventis: Consultancy; Ascentage: Other; Genfleet: Other; BMS: Other: Grant/research support. DiNardo: Agios/Servier: Consultancy, Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; ImmuneOnc: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Takeda: Honoraria; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; Forma: Honoraria, Research Funding; Novartis: Honoraria; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding. Konopleva: Sanofi: Other: grant support, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; Cellectis: Other: grant support; Calithera: Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; Agios: Other: grant support, Research Funding; Ablynx: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; Stemline Therapeutics: Research Funding; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; KisoJi: Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Rafael Pharmaceuticals: Other: grant support, Research Funding; Forty Seven: Other: grant support, Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding. Daver: Hanmi: Research Funding; Genentech: Consultancy, Research Funding; Novimmune: Research Funding; Astellas: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Novartis: Consultancy; Trovagene: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; FATE Therapeutics: Research Funding; Amgen: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Glycomimetics: Research Funding; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. Ravandi: Astex: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Taiho: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Novartis: Honoraria; Amgen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Prelude: Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding; AstraZeneca: Honoraria; Xencor: Honoraria, Research Funding. Pemmaraju: LFB Biotechnologies: Consultancy; Sager Strong Foundation: Other; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Consultancy; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Aptitude Health: Consultancy; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; Protagonist Therapeutics, Inc.: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Affymetrix: Consultancy, Research Funding; MustangBio: Consultancy, Other; Roche Diagnostics: Consultancy; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Springer Science + Business Media: Other; Clearview Healthcare Partners: Consultancy; CareDx, Inc.: Consultancy; Cellectis S.A. ADR: Other, Research Funding; Daiichi Sankyo, Inc.: Other, Research Funding; Plexxicon: Other, Research Funding; Samus: Other, Research Funding; DAVA Oncology: Consultancy; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy; Bristol-Myers Squibb Co.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy. Short: Novartis: Honoraria; NGMBio: Consultancy; Jazz Pharmaceuticals: Consultancy; Astellas: Research Funding; AstraZeneca: Consultancy; Takeda Oncology: Consultancy, Research Funding; Amgen: Consultancy, Honoraria. Issa: Novartis: Consultancy, Research Funding; Kura Oncology: Consultancy, Research Funding; Syndax Pharmaceuticals: Research Funding. Takahashi: Celgene/BMS: Consultancy; GSK: Consultancy; Novartis: Consultancy; Symbio Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kantarjian: Astra Zeneca: Honoraria; Aptitude Health: Honoraria; Ascentage: Research Funding; Pfizer: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Astellas Health: Honoraria; Ipsen Pharmaceuticals: Honoraria; Jazz: Research Funding; Precision Biosciences: Honoraria; Amgen: Honoraria, Research Funding; KAHR Medical Ltd: Honoraria; NOVA Research: Honoraria; AbbVie: Honoraria, Research Funding; Immunogen: Research Funding; Daiichi-Sankyo: Research Funding; BMS: Research Funding; Taiho Pharmaceutical Canada: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal